- Enhancing chemotherapy – a ‘Trojan horse’ cancer treatment
- Turning cancer cells into fat could bring hope to millions of people
- Immunotherapy is gaining credence as a viable cancer treatment
- MuTaTo might be the cure we need – but is it too good to be true?
- The Tumor Monorail
- DNA nanobots destroy cancerous cells by shutting off their blood supply
- Could the future be cancer-free?
Our body is made from trillions of cells, forming tissues and organs. And just like the bodies they form, cells have a specific pattern based on which they grow, divide, and eventually, die. But some mutations, either inherited or developed over time, can make cells grow rapidly and out of control. That’s how tumours form, and unfortunately, some of them are malignant. Malignant tumours – or cancers – can appear in one part of the body, and in some cases, spread to other parts of the body very quickly in a process known as metastasis.
According to the World Health Organization, cancer was responsible for 9.6 million deaths in 2018, which makes it the second leading cause of death worldwide.
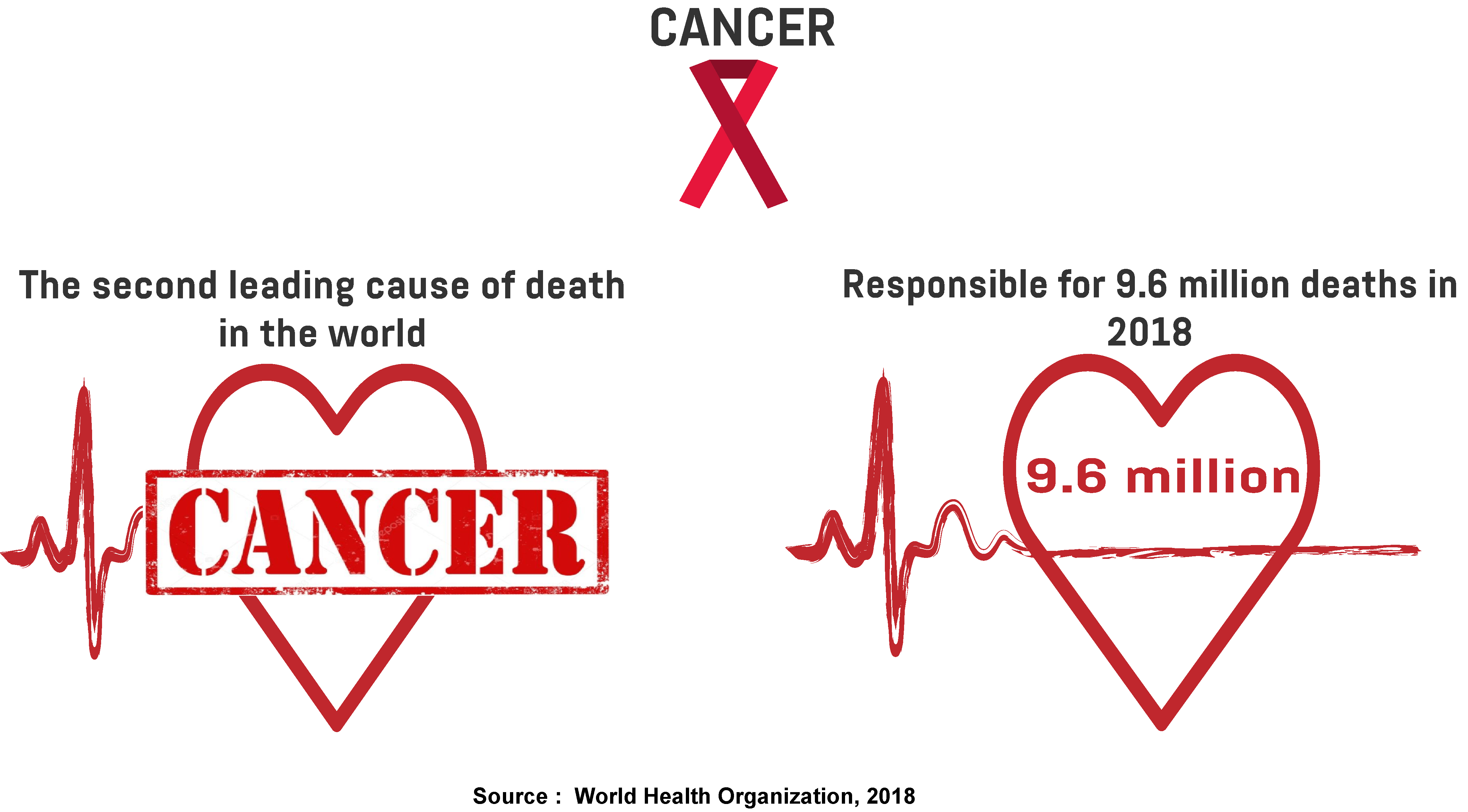
The most common types of cancer are lung, breast, and bowel cancer. Depending on the type of cancer, the mortality rate can be reduced if it’s identified at an early stage. But the treatment is complex and accompanied by high medical expenses. In fact, estimates show the world spent $133 billion on cancer medicine in 2017, while the average price of a new drug was $150,000. Moreover, 20 per cent of total cancer spending in 2017 came from the EU, while 45 per cent was from the US.
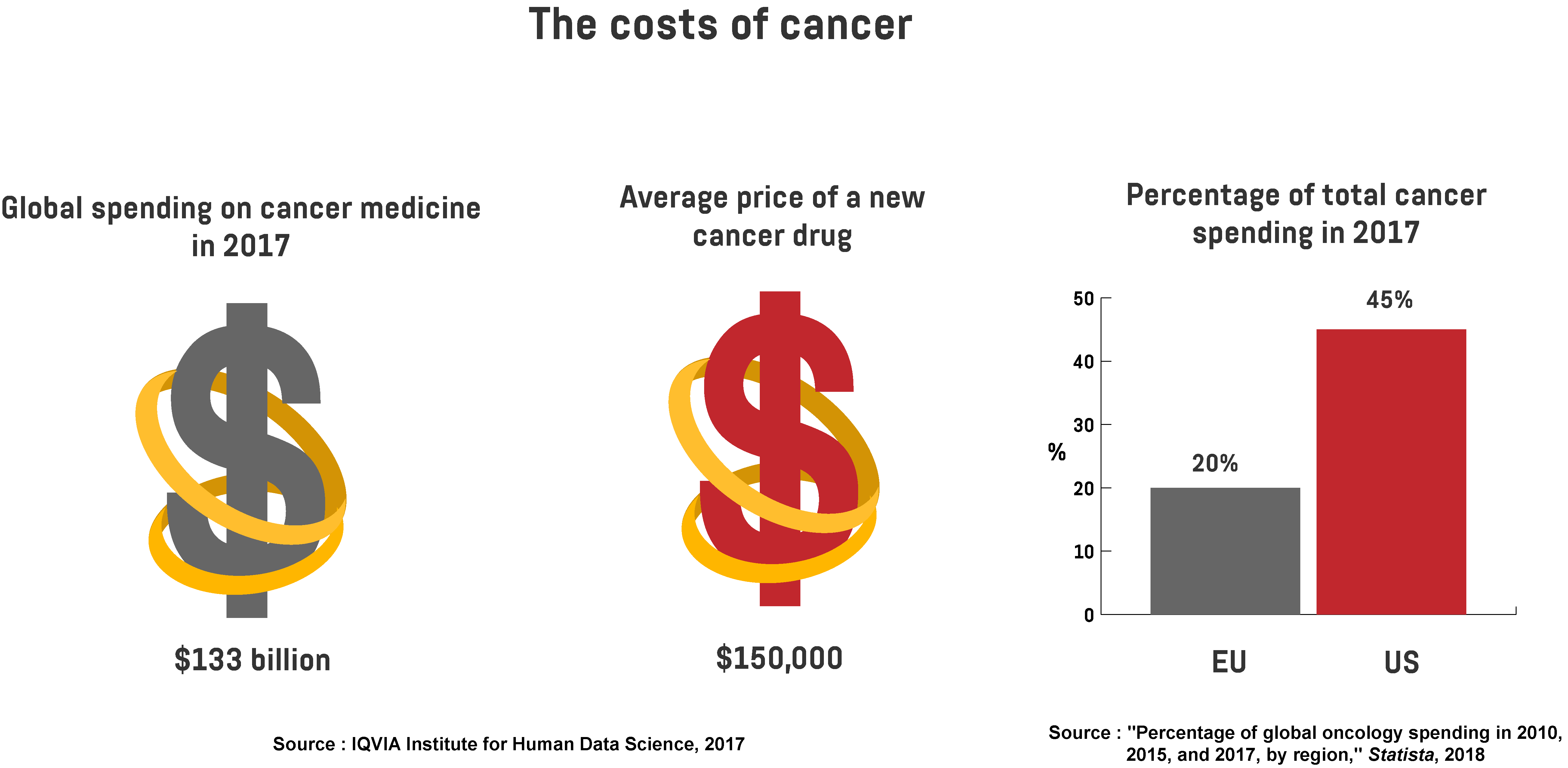
In a survey conducted by the non-profit organisation Kaiser Family Foundation, 72 per cent of US residents agree that drug costs for cancer treatment are quite “unreasonable”. Besides being expensive, existing cancer treatments are unpredictable, too, and don’t guarantee a positive outcome.
To solve these challenges and improve cancer treatment effectiveness, scientists have put a lot of effort into cancer research. And recently, they’ve made some major breakthroughs in this field, offering great potential to change the future of healthcare.
Enhancing chemotherapy – a ‘Trojan horse’ cancer treatment
One of those breakthroughs was achieved by a team of researchers from the Institute of Cancer Research (ICR) and The Royal Marsden NHS Foundation Trust. They created a drug called tisotumab vedotin (TV) that “combines a chemotherapy agent with an antibody” and acts like a ‘Trojan horse’, attacking cancer cells from the inside. The antibody connects to the cancer cell and forces it to ‘let it in’. Once the drug is drawn in, it attacks the cell from the inside and destroys it. To prove its effectiveness, the researchers tested the drug on 147 patients who suffer from different types of cancer. Most of the participants were in the advanced stages of cancer, and their bodies showed resistance to earlier treatments.
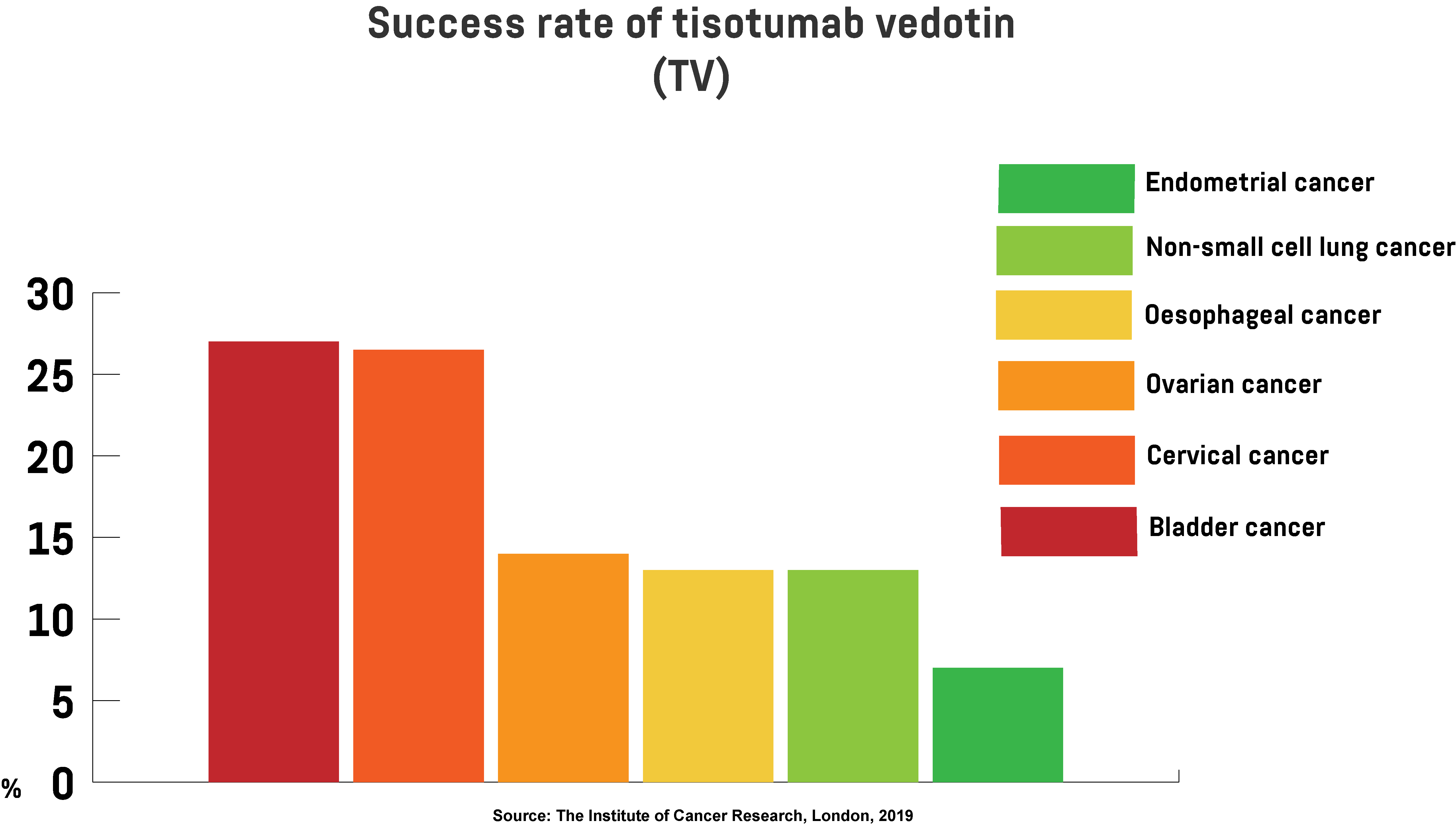
The results of the testing, published in the journal The Lancet Oncology, show that some participants responded well to the drug and their cancer cells shrunk or stopped growing completely. For instance, it’s been revealed that TV had a positive effect on 27 per cent of patients with bladder cancer, 26.5 per cent with cervical cancer, 14 per cent with ovarian cancer, and 7 per cent of participants with endometrial (uterine) cancer.
The positive effect lasted for 5.7 months on average, while for some patients, it lasted for 9.5 months, until it eventually faded away. However, the treatment did cause some side effects, such as nausea, fatigue, and nose bleeds, but as Professor Johann de Bono, who took part in the research, notes, such side effects were all manageable. Based on the findings, the team proved their newly developed drug has great potential to treat patients with different types of cancer, including those with low survival rates. The team has started another trial session that includes patients with lung and pancreatic cancer.
Turning cancer cells into fat could bring hope to millions of people
Significant progress in cancer treatment research had also been made by scientists from the University of Basel in Switzerland, who found a way to convert breast cancer cells into harmless fat. Breast cancer is the most common cancer type among women. Every year, 2.1 million women are diagnosed with it, while 627,000 of them died in 2018. Most deaths happen after cancer cells start to invade other parts of the body during metastasis. The study, conducted on mice, reveals how the scientists used the plasticity and adaptability of breast cancer cells during metastasis to transform those cells into fat. As cancer cells metastasise, they experience a change dubbed epithelial-mesenchymal transition (EMT), in which they become adaptable and resemble stem cells. What the scientists propose is using this state to turn cancer cells into a harmless cell type.
To prove their hypothesis, they injected breast cancer cells into female mice. Then they treated the mice with two drugs, rosiglitazone, which is used to treat type 2 diabetes, and trametinib, an anti-cancer drug, both of which are already approved by the US Food and Drug Administration (FDA). After using these two drugs together, the scientists discovered that cancer cells that have started to metastasise turned into fat cells similar to normal fat cells that can’t divide and grow. This combination of drugs didn’t turn all the cancer cells into fat cells, but it did stop further metastasis. In the future, the scientists are planning to test their breakthrough together with traditional chemotherapy treatment to prevent cancer cells from growing and entering metastasis. They would also like to investigate what kind of an effect this treatment would have on other types of cancer.
Immunotherapy is gaining credence as a viable cancer treatment
The latest breakthroughs in cancer treatment bring a lot of hope to patients suffering from this deadly disease. One of the recent advances is based on using the body’s immune system to fight cancer cells. The treatment, expected to enter human trials this year, was developed by researchers at the Francis Crick Institute in London. They believe that using cells from the immune system of healthy people and implanting them in cancer patients could enhance the body’s ability to fight cancer. This could even increase the ten-year cancer survival rate from 50 to 75 per cent.
Using the immune system to fight cancer involves no chemicals or drugs, and it doesn’t come with side effects like in traditional chemotherapy treatments. So, instead of bombarding the patient’s body with chemotherapy drugs, doctors could upgrade it with disease-fighting cells. One of the researchers involved in the project, Charlie Swanton, is confident that in the next two decades, our immune system could be a great weapon in treating various types of cancer. What the researchers envision is creating ‘banks’ to store healthy immune cells, allowing doctors to order and implant them into patients’ bodies in just a few hours. This is much more effective than existing immunotherapy drugs, because such drugs not only attack cancer cells, but can also negatively affect healthy cells. Moreover, since cancer develops quickly and it’s hard for pharmaceutical companies to keep up, this “ultimate do-it-yourself approach” could be a better alternative.
MuTaTo might be the cure we need – but is it too good to be true?
While many of these discoveries are still in the early stages, an Israeli pharmaceutical company called Accelerated Evolution Biotechnologies (AEBi) might have taken a step further, claiming it will have a complete cure for cancer within a year! The company’s anti-cancer drug is called MuTaTo, which stands for multi-target toxin. MuTaTo uses peptides (chains of amino acids), which are cheap and easy to produce, to carry a strong toxin to destroy cancer cells. While most drugs used in cancer treatment attack a specific target in the cell, one at a time, MuTaTo works differently. “Instead of attacking receptors one at a time, we attack receptors three at a time – not even cancer can mutate three receptors at the same time,” says Dr. Ilan Morad, the CEO of AEBi. To defend themselves, cancer cells use detoxification mechanisms to pump out the toxin. Detoxification, however, takes time. And the researchers believe that when the toxin is strong, it could kill the cell even before detoxification begins.
The plan is to make this treatment personalised, because not every patient responds to anti-cancer drugs in the same way, and no two tumours are the same. So, each patient will be required to give a biopsy for analysis in the lab, after which the doctor will know the right “molecule cocktail needed to cure [the] disease”. Since such a ‘cocktail’ could kill cancer cells, it means that the patient would end the treatment after just a few weeks. So far, AEBi’s researchers have conducted the first experiments on mice, in which the drug was able to prevent the growth of cancer cells without disrupting healthy cells. The researchers will soon begin clinical trials, and they’re confident they will bear fruit and make the treatment available for specific cancer cases.
The Tumor Monorail
Another game-changing solution has recently gained “breakthrough device” status from the FDA. The ‘Tumor Monorail’, as it’s called, was developed by scientists from Georgia Tech and Emory University. The concept, revealed in the journal Nature Materials in 2014, is designed to make aggressive brain tumours, such as glioblastoma, more manageable. Glioblastoma is a severe type of cancer that occurs in the brain or spinal cord. It can occur at any age, but it’s more common among the older population. This brain tumour is very difficult to treat, which is why having a glioblastoma diagnosis usually equates to getting a death sentence. But the Tumor Monorail could help glioblastoma patients to win the battle.
The device is shaped like a long tube with a container at the end. What this innovation is designed to do is imitate the structure of the brain’s white matter to ‘lure’ cancer cells into the container instead of spreading across the brain and affecting healthy tissue. When needed, neurosurgeons can access the container to empty it and remove the collected cells. The device has been previously tested on rats, and during the experiment, the Tumor Monorail lured cancer cells into the container, which was filled with a toxic substance. As a result, the spread of cancer cells was slowed down and the tumour was reduced by over 90 per cent. This was “the first demonstration of bringing the tumor to your drug rather than your drug going into the brain and killing valuable cells,” explains one of the researchers, Ravi Bellamkonda. The team is optimistic they’ll get approval to test their innovation on human patients by the end of this year.
DNA nanobots destroy cancerous cells by shutting off their blood supply
An international team of researchers, including from China, Australia, and the US, demonstrated how nanobots could be used to fight various forms of cancer. Each nanobot is made from a DNA sheet folded into a tube, containing a blood-clotting drug called thrombin. Commonly used to treat bleeding, thrombin restricts blood flow within blood vessels. When the blood supply is cut off, cancer cells can no longer grow, and the cancerous tissue will eventually die. As for normal tissue, the nanobots are perfectly safe, and there’s no evidence that these tiny bots could cause any side effects.
The innovation has been tested on mice, and the results were published in the journal Nature Biotechnology. As the researchers observed, within 24 hours of the treatment, the blood vessels that were feeding the primary tumour had been cut off, while over the next 72 hours, the tumour started to die. This technique extended the life of the mice to 45 days. In contrast, the life expectancy of non-treated mice was 20.5 days. “We have developed the first fully autonomous, DNA robotic system for a very precise drug design and targeted cancer therapy,” says Professor Hao Yan, the director of the Arizona State University Biodesign Institute’s Centre for Molecular Design and Biomimetics, adding that “this technology is a strategy that can be used for many types of cancer, since all solid tumour-feeding blood vessels are essentially the same.” Although the testing has only been conducted on animals so far, he thinks the team is “much closer to real, practical medical applications of the technology”.
Could the future be cancer-free?
Cancer is, without a doubt, one of mankind’s biggest health challenges. The number of people who get diagnosed with cancer is rapidly growing, and every year, this severe illness takes millions of lives around the world. Over the past few years, scientists have been working really hard to make progress in cancer treatment research, and they’ve come up with some pretty remarkable breakthroughs. Recent advances could make cancer diagnostics and treatment more effective and less expensive, increasing patients’ survival rate. Although we’re still waiting for a ‘miracle cure’ that will finally put an end to cancer, if we take these new discoveries into account, we might be one step closer to a cancer-free future.
Just imagine, after billions of dollars and so many years spent on cancer research, we could take control of this disease. Breast, brain, and any other type of cancer would no longer be a deadly disease. In the future, doctors could devise nanobots that track down cancer cells and destroy them. Perhaps we’ll even make use of gene editing to eliminate the genetic mutations that cause cancer. With each new breakthrough in this field, we’re getting closer to this future.
Share via:


