What do you think about gene editing? Tell us in this survey!
Who wouldn’t want to be a healthy 150-year old? It may sound like science fiction, but it could soon be possible, thanks to gene editing. Many people believe that gene editing is the future, especially now that we have CRISPR gene editing technology. The truth is, gene editing is becoming easier and it will be used more and more often. Since it will play an increasingly important role in our lives, it is crucial that all of us get a better understanding of the technology. Let’s start at the beginning.
Our DNA, our genes, and our proteins
Genes are a part of our DNA, the genetic code we inherit from our parents. It is located in the nucleus of every cell in our body. It is made up of small units that form the code for all the ‘machinery’ our cells need to survive. We get fifty percent of our DNA from our father and the other half from our mother. The cellular machines are called proteins. Each piece of genetic code for each individual protein is called a gene. Humans have at least 19.000 genes, although the exact number is still under debate.
Genes code for proteins. The functions of proteins differ. Some proteins create energy, while others move to the blood, functioning as signals for other parts of the body. Some proteins even create other proteins by reading the genetic code. The genetic code can contain errors, also called mutations. To prevent errors in the proteins – proteins with errors cannot function properly – it is essential that the genetic code is error-free. Fortunately, we also have proteins that check our DNA for mistakes and fix them.
Even though cells have all this machinery to keep the genes error-free, mutations sometimes do creep in. Not all mutations pose problems, but there are those that can cause diseases. In the process of gene editing, researchers and doctors exchange (part of) a gene to fix a disease-causing mutation.
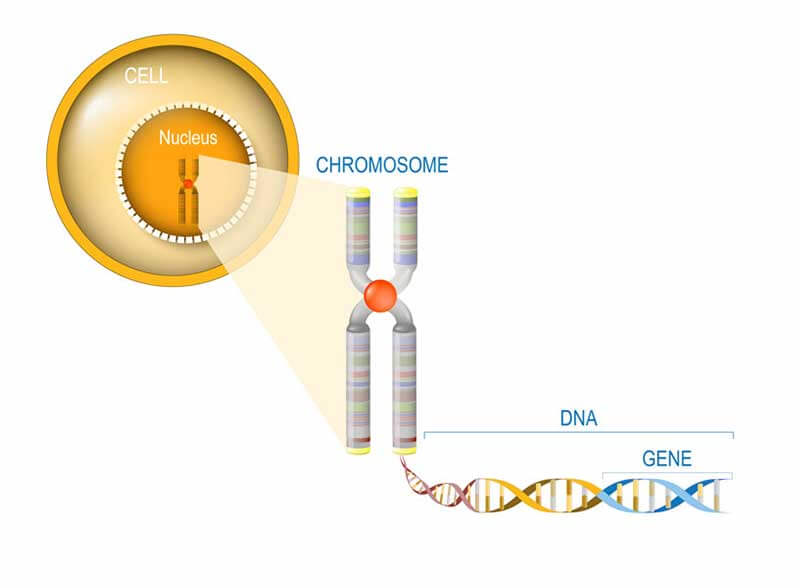
Errors in genes cause diseases
Genetic mutations lie at the centre of many diseases. Some mutations are inheritable, meaning that if your parents have a mutation, they pass it on to you. Disease-causing mutations can, however, also skip a generation, which means the genetic disease is transferred to you, without it affecting your parents.
Inheriting a disease
Huntington’s is a good example of an inheritable disease. When one of your parents has it, you have a fifty percent chance of getting it as well. This neurodegenerative disease causes cells in your brain to slowly die off. The Huntington’s Disease (HD) gene is responsible for this and codes for the huntingtin protein. If you have the mutation, the gene and the protein are longer than they should be, causing the protein to be toxic for the brain. Everyone with this gene expansion will get the disease, which will be fatal. The ‘upside’ is that symptoms only appear around the age of 40, which means there is a lot of time to replace this faulty gene with the shorter, healthy variant. This could eradicate Huntington’s disease forever.
Stopping cancer
Cancer has also caused great problems due to genetic errors. Cancer develops because several mutations, in several different genes, pile up in the DNA. Imagine if you could get the DNA of a tumor tested and replace all the mutated genes in your body with healthy ones. The tumor could then be removed, without increased chances of recidivism. Even undiagnosed metastases could be prevented from growing any further, since their mutations would also be restored during the body-wide gene editing process.
Extending life
One more extremely promising aspect of gene editing is life extension. Extensive research indicates that the genes affecting lifespan are involved with sensing the amount of energy present in our bodies. Having less energy present, leads to activity changes in those genes that increase lifespan. It is also well-known that eating less – thus having less energy present – extends life. Using gene editing, we can increase and decrease the activity of certain genes involved in aging, in such a way that lifespan is increased. This has already been done in worms, yeast, flies, and mice – with surprising results. Some worms live six times longer and mice can live up to twice as long. This shows that lifespan can be increased by influencing the activity of these genes. More importantly, the genes that are responsible for this in worms, flies, and mice are also present in humans. That means that humans can potentially also be treated to live a longer healthy life. Imagine getting a short gene editing therapy at age 25 and then living to 150 or more in good health.
Editing genes with CRISPR-Cas9
The CRISPR-Cas9 technique was recently discovered in bacteria, where it serves as a protective mechanism against viruses. The system makes use of the Cas9 protein, which can cut DNA. You could see it as a pair of molecular scissors that prevents a virus from replicating. Cas9 binds a small molecule called guide RNA, which not only looks like DNA, but can also bind to it. As Ellen Jorgensen, molecular biologist and co-founder of the world’s first community biolaboratory, describes it in her TED talk below: “The guide RNA forms the leash for the Cas9 molecule, that keeps the Cas9 out of the genome, until it finds the specific place where it needs to cut”.
Scientists can design this guide RNA to specifically target a gene of interest, say the HD gene. When it binds, Cas9 cuts the DNA to remove the mutated HD gene. The cell will try to repair the cut by putting it back together. Scientists can instruct the cell to incorporate the healthy HD gene to cure the disease. This technique can be applied to all genes in the DNA and it gives scientists control over which genes work and which don’t.
Since Cas9 is a protein, there is a piece of DNA – a gene – that codes for it as well. This means that the machine and the guide RNA can be inserted into the DNA of any cell with the use of existing techniques. When the technique is flawless, it could be applied at all stages of life, from a fertilised egg to a 70-year-old person.
Plants also have genes
It’s not just humans and animals who have DNA and genes. Plants do, too. We use plants for a variety of reasons, but they mainly provide us with fruits and vegetables for consumption, which gives our bodies healthy and essential nutrients. Genes in the plant’s DNA code for most of the healthy substances that we get from them. Using genetic modification, scientists can add more nutrients to our crops. This is called biofortification. A perfect example of biofortification is the so called ‘golden rice’. It gets its name from its golden colour, which is the result of beta-carotene, a compound that scientists engineered into the rice to make it healthier. Our body converts beta-carotene into vitamin A.
The development of this rice is important because vitamin A deficiency (VAD) affects about a third of the children under five worldwide and is the main cause of childhood blindness. Administration of vitamin A supplements is costly and inefficient. However, scientists found that just 72 grams of dry golden rice a day is enough to prevent VAD. As rice is a staple in most countries where VAD is prevalent, supplementing this way is a cheap and easy solution. With gene editing we can add all types of nutrients to all types of fruit and vegetables. We could ensure that everyone – not only people in third-world countries – leads a healthier life.
Ethics of gene editing
One of the biggest problems with gene editing, especially in humans, is the controversy surrounding it. Some people believe that we shouldn’t play around with genes, while others see no problems or limitations. For example, we could cure somebody’s colourblindness. This way, he or she can see the entire spectrum of colours again, probably improving their life. We could also expand the sight of healthy individuals by editing in additional colours. This does not cure anything, but some people might be very keen to have this done.
We can, however, do many more useful things with these techniques. For example, antiviral or anti-bacterial CRISPR-Cas9 systems could be inserted into the genome of young children. As soon as they would be infected with a virus or bacterium, their CRISPR-Cas9 vaccination could prevent those viruses or bacteria from surviving and thriving, creating an artificial form of immunisation. Something like this has already been tested as a treatment and prevention for HIV. If this works, it will pave the way for genetic vaccinations.
But we could also check the DNA of our unborn babies for genetic abnormalities. Do we then want to alter their genome? Let’s say the baby has the Huntington mutation. We could fix that, but while working on the baby’s DNA, we could also change its hair and eye colour, and make the baby a little stronger by enhancing its muscle development. It is important to note the ethical and biological difference of genetically editing egg or sperm cells and any other type of cell. The main distinction between the two is that the former will change the DNA for generations to come, while the latter is just for one person’s lifetime. Both have separate ethical issues that need to be considered. Numerous people, from inside as well as outside the scientific community, have warned against the consequences of CRISPR, if hastily implemented for human gene editing.
The intentions might be good, but it might also increase inequality, as gene editing will, most likely, initially only be accessible to the rich. “Once you start creating a society in which rich people’s children get biological advantages over other children, basic notions of human equality go out the window,” David King, former molecular biologist and founder of Human Genetics Alert, warns in the Guardian.
In other words, gene editing holds the future, but only if it is properly managed and controlled. We need regulation and we need committees to guide research and development in the right direction. Only then can we truly be prepared for the future of gene editing.
Share via:


